Pharmaceutical isolators have become a cornerstone of modern pharmaceutical automation. As regulatory expectations rise and product sensitivity increases, isolators play a critical role in maintaining sterile conditions while protecting both the product and operating personnel. They are widely used across aseptic processing, oral solid dosage production, sterile handling operations, potent API containment, and hazardous substance management within cleanroom automation environments.
The fundamental purpose of pharmaceutical isolators is to create a controlled, sealed environment where critical pharmaceutical process control activities can be executed without external contamination. However, maintaining sterility, pressure balance, and environmental stability within an isolator is complex. Manual systems or fragmented process automation approaches introduce variability and increase compliance risk. This is where industrial automation and PLC automation become essential.
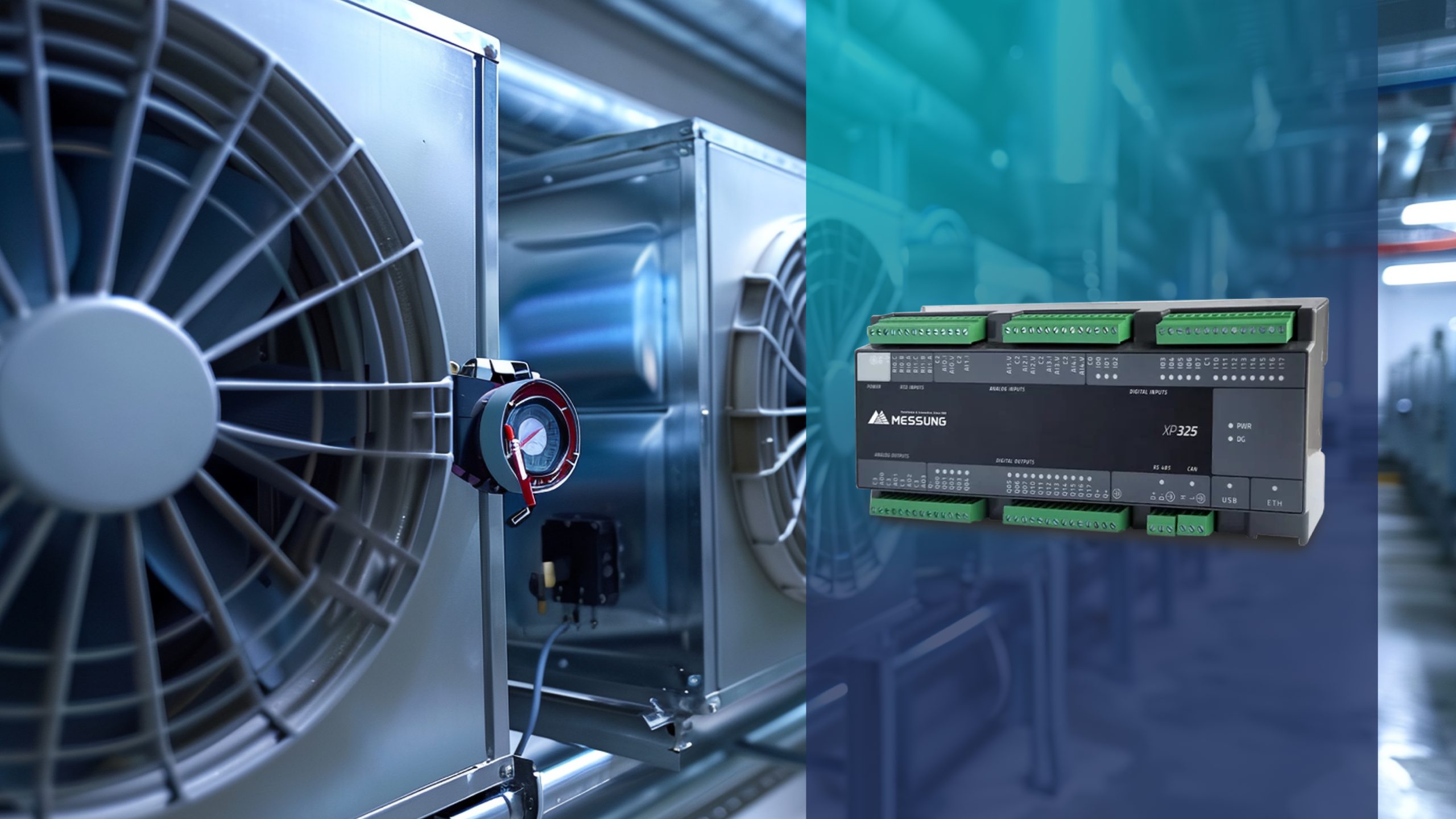
Messung’s NX-ERA XPRESS XP-325 programmable logic controller, paired with an HMI, provides a structured PLC-based control system for pharmaceutical isolator applications. Designed as a compact PLC for small and mid-sized machines, the XP-325 offers reliable process control automation while meeting the demands of regulated cleanroom monitoring systems.
Purpose of Automation in Pharmaceutical Isolators
Automation in pharmaceutical isolators is not simply about convenience. It is a fundamental requirement for pharmaceutical automation to ensure sterility, safety, and repeatability. A closed loop control system maintains sterile barrier integrity while ensuring accurate environmental monitoring in pharma environments. Automated compliance reporting becomes possible through continuous real time process monitoring of pressure, temperature, and humidity. Deviations are detected instantly, alarms are generated automatically, and events are recorded through data logging PLC functions. This minimizes operator dependency and strengthens pharmaceutical process control.
Automation systems also support secure electronic records that align with regulatory expectations such as 21 CFR Part 11. Seamless integration with a SCADA system or MES allows isolator operations to function as part of a larger pharma automation solution, enabling centralized visibility and traceability.
System Overview
In a pharmaceutical isolator automation system, the NX-ERA XPRESS XP-325 PLC functions as the central programmable controller. As an industrial PLC, it coordinates field devices, executes control logic, and ensures predictable system behavior under all operating conditions. Operator interaction is managed through an HMI, providing clear visibility into isolator status and process conditions. The system integrates pressure sensors, airflow and velocity sensors, environmental monitoring instruments, and glove integrity test modules. Automated decontamination cycle control ensures repeatable and validated operation. Door interlocks, chamber interlocks, material pass-boxes, exhaust systems, and utilities are managed through a unified PLC control system. This eliminates isolated subsystems and creates a fully integrated process automation architecture based on intelligent controllers.
Key Automation Components
Environmental Monitoring
Environmental monitoring in pharma isolators is critical for contamination control. The automation system continuously monitors differential pressure across HEPA supply, chamber, and exhaust zones using cleanroom monitoring systems.
Temperature and humidity sensors provide real-time feedback, while airflow velocity sensors ensure proper laminar flow conditions. Filter clog status is monitored using differential pressure signals, supporting proactive PLC maintenance planning. These parameters form the foundation of pharmaceutical process control and automated environmental stability.
Fan and Blower Control
Supply and exhaust fans are managed through coordinated control logic to maintain defined pressure relationships within the isolator. This closed loop control system prevents cross-contamination and protects operator safety.
Cascade pressure control ensures stable containment during all operating modes. The integration of these functions within a PLC-based control system enhances consistency and reduces operational risk.
Decontamination Cycle Automation
Decontamination is a critical requirement in pharmaceutical isolators. The XP-325 PLC manages decontamination cycle sequencing, including vapor generation, nozzle actuation, and timing control.
Temperature, humidity, and concentration parameters are monitored continuously during the cycle. Automated sequencing ensures each cycle follows the same validated process, supporting pharmaceutical automation and compliance readiness.
Glove Integrity Test
Glove integrity testing is an essential safety requirement. The automation system supports automated pressure decay testing, where leak integrity is verified through controlled test sequences.
Test results are recorded electronically using data logging PLC functions. This supports traceability, audit readiness, and long-term compliance documentation.
Door and Safety Interlocks
Safety PLC concepts are applied through comprehensive interlocking logic. Door position sensors detect open and closed states, preventing unsafe operations that could compromise sterility.
Emergency stop logic and safe shutdown routines ensure predictable system behavior under abnormal conditions, reinforcing the role of the PLC as a safety-oriented programmable controller.
HMI and Operator Interface
The HMI serves as the primary operator interface for isolator control. Operators can select recipes for sterile operations, containment processes, or decontamination cycles.
Real-time displays provide visibility into pressure, temperature, and humidity trends. Alarm dashboards support quick fault identification, while batch logs ensure structured operation. User-based access control strengthens automated compliance reporting and regulatory alignment.
Communication and Data Handling
Reliable communication is essential for modern automation systems. The PLC and HMI communicate over Ethernet, enabling fast data exchange.
OPC UA connectivity allows seamless integration with SCADA systems and higher-level pharmaceutical automation platforms. Real-time process monitoring data, alarms, and cycle reports are transferred continuously. Optional Modbus communication enables flexible integration within existing industrial automation architectures and IoT systems.
Compliance and Safety Features
Pharmaceutical isolator automation must support regulatory compliance by design. The system enables electronic records, audit trails, and controlled access aligned with 21 CFR Part 11 requirements.
Designed for GMP environments, the automation architecture emphasizes contamination prevention and process integrity. Calibration reminders and maintenance logs support long-term reliability and structured PLC maintenance practices.
Benefits of Automating Isolators with NX-ERA XPRESS XP-325 PLC
Automating pharmaceutical isolators with Messung’s NX-ERA XPRESS XP-325 PLC delivers measurable benefits:
● Higher sterility assurance through controlled environmental monitoring
● Reduced cross-contamination risk using pressure-based closed loop control
● Real-time visibility into critical parameters
● Reduced operator exposure to hazardous substances
● Improved repeatability of decontamination cycles
● Centralized control with complete traceability
These outcomes position the XP-325 as a smart PLC solution for critical pharmaceutical automation applications.
Conclusion
Pharmaceutical isolators demand automation systems that are reliable, precise, and compliant. Messung’s NX-ERA XPRESS XP-325 PLC, combined with an HMI, delivers a dependable PLC-based control system for isolator automation.
Through precise environmental control, automated decontamination, intelligent interlocks, and structured data handling, the XP-325 supports product quality, operator safety, and regulatory compliance. It stands as a confident industrial automation platform for modern pharmaceutical isolator systems.